usp class vi pdf
Class VI testing is aimed to. EPDM 70-291 FDA USP Class VI 0154 EPDM 75 A thickness.

Gauge Guard Protector Literature Resources Rubber Fab
7 USP Class VI materials EPDM silicone fluorocar-bon and perfluoroelastomer 24 materials which are compliant to FDA 21 CFR1772600 Specially formulated for long term.

. USP Class VI plastic. S-2013-01361SAMi released 26th July 2013 for - Biological reactivity test in vivo - Systemic. 12072019 hardness DIN ISO 7619-1.
Intracutaneous Test are used for elastomeric materials espe-1 USP High-Density Polyethylene RS. USP Class VI refers to a set of biocompatibility testing requirements from the US. Used to classify plastics in Classes I - VI based on end use type and time of exposure of human tissue to plastics of which Class VI requires the most stringent testing of all the six classes.
Pharmacopoeia USP Class VI outlines requirements for system toxicity and intracutaneous toxicity for these cleaner compounds. 75 5 Shore A color. The USP Class VI compounds must be.
There are six classes VI being the most rigorous. This information has been carefully prepared to help in selecting the cor-rect elastomer or. Class VI Test - USP USP 09 Sodium Chloride for Injection NaCl Cottonseed Oil CSO 1 in 20 Ethanol in NaCl EtOH and Polyethylene Glycol 400 PEG 70 2 oc for 24 2 hours.
Interim Revision Announcement 2 87 Biological Reactivity Tests In Vitro Official November 1 2015 NaCl or serum-free mammalian cell culture media as Ex-Table 1. USP Class testing is one of the most common methods of testing to determine bio-compatibility of materials. 1 -6 mm Datum.
USP USP class VI was especially devel-oped for the pharmaceutical industry. Results of tests are stated in the following Test Reports. Summary of in vivo and in vitro.
USP Class VI - Konformitätserklärung USP Class VI - Declaration of Conformity USP Class VI - Declaration de conformite ifm electronic gmbh Friedrichstraße 1 45128 Essen Germany E-Mail. Cially to elastomeric closures for which the appropriate Bio-Table 1. USP Class VI and Biocompatible Rubber.
USP 4 extracts Requirements satisfied Implantation test USP Disc of test sample with Ø10mm and thickness 1 mm Requirements satisfied Table 1. Pharmacopeia USP a non-profit. USP Class VI testing is conducted by producing an extract of the product with different extraction fluids such as polyethylene glycol and vegetable oil and injecting it in.
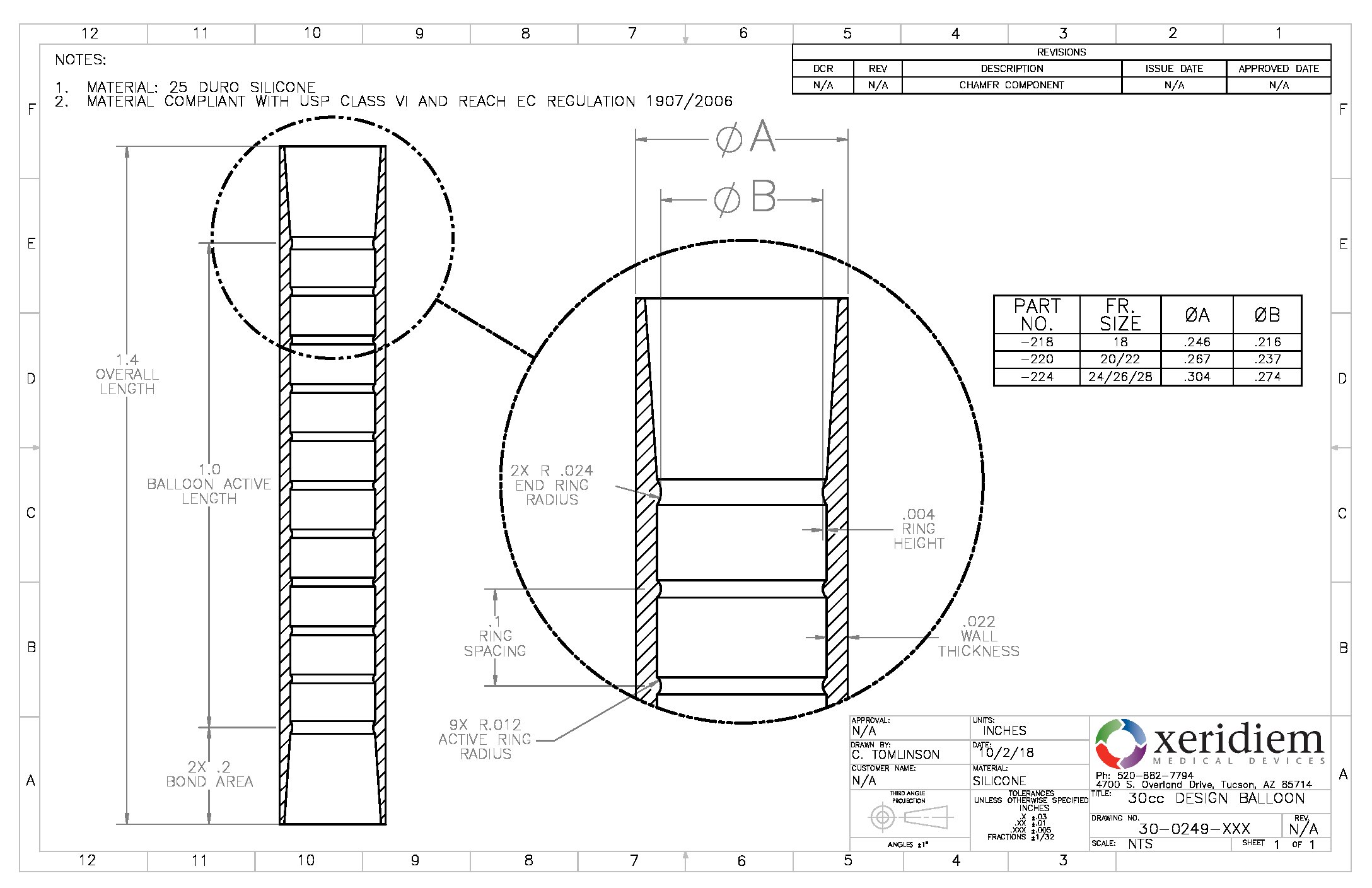
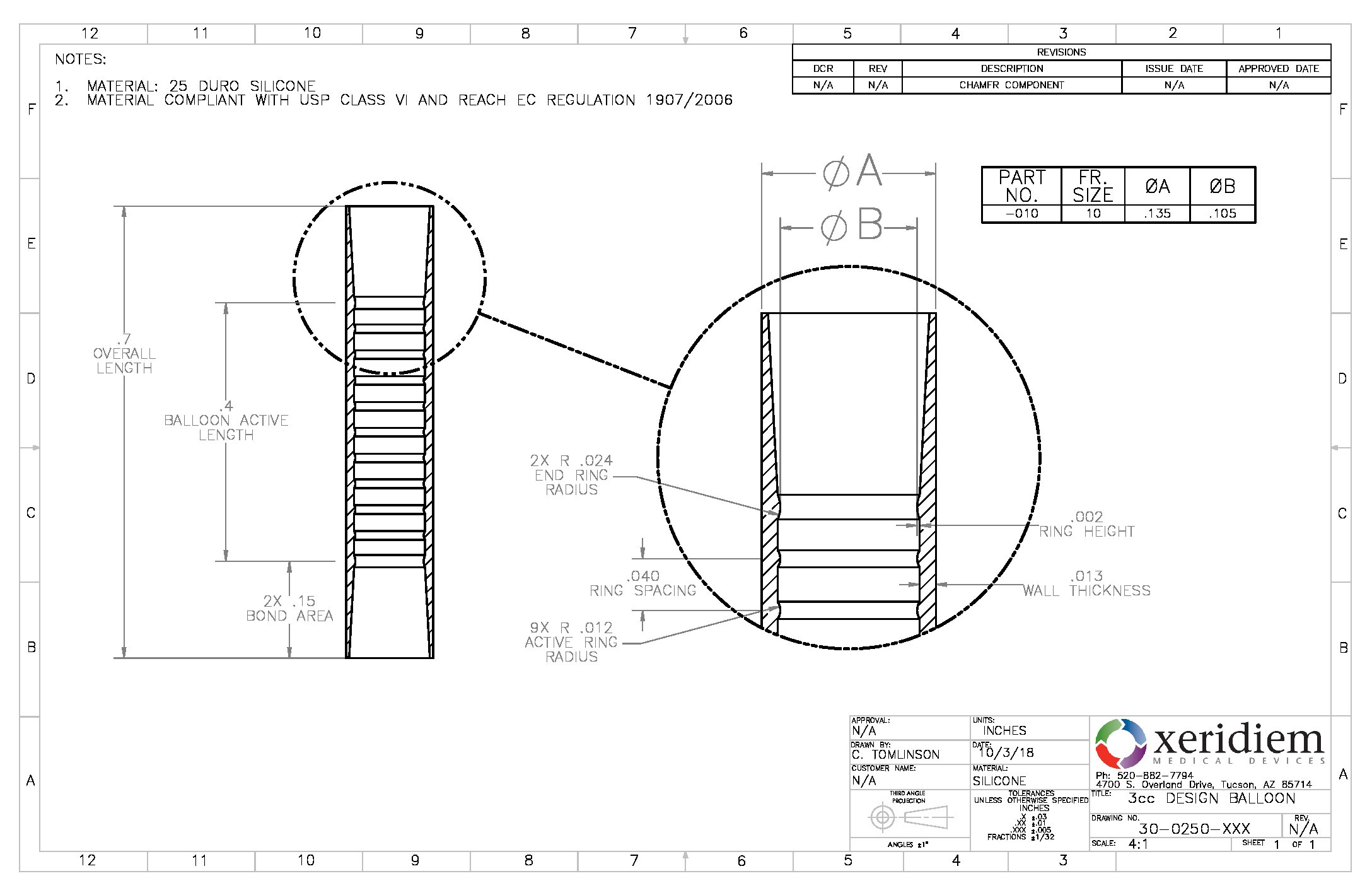
Balloon Silicone 7 7mm Od Spherical Quantity 10 Bag

Sanitary Gasket Material Guidelines Selection Rubber Fab

Usp Class Vi Foster Corporation

Keofitt World Leaders In Sterile Sampling
Lg Tone Free Fp7c Plug Wireless True Wireless Bluetooth Uvnano Earbuds Tone Fp7c Lg Usa

Microvantage Mpa Series Shelco Filters Pdf Catalogs Technical Documentation Brochure

Iso 10993 Vs Usp Class Vi Medical Molding And Bicompatible Rubber The Rubber Group
![]()
Usp Class Vi Silicone Is Independently Certified For Biocompatibility Specialty Silicone Products Inc

Usp Class Vi Gaskets Seals Usp Class 6 O Rings Ppe
Usp31nf26s1 C1031 General Chapters 1031 The Biocompatibility Of Materials Used In Drug Containers Medical Devices And Implants

Pdf Brochure And Data Sheet On The Pure Fit Spt 50 Sti

Skr Filters Sterile Point Of Use Small Batch Liquid Gas Filtration

Aktaprocess Ge Healthcare Life Sciences Pdf Catalogs Technical Documentation

Isolast Plus J9515 J9516 Trelleborg Sealing Solutions Pdf Catalogs Technical Documentation Brochure

Formlabs Biomed Clear Resin Cartridge 1l Dynamism